Ph Value of Ethanoic Acid
How do you calculate this thing. Further addition of such a small amount as 001 mL of the alkali raises the pH value by about 3 units to pH 7.

What Is Ethanoic Acid The Chemistry Blog
Note the colour chart on the bottle or package.
. The pH of a 001M solution of HCl is equal to log 10 001. Ethanoic acid and ammonia solution. Now if we know the value for K a we can calculate the hydrogen ion concentration and therefore the pH.
Remember that we want to calculate the pH of a buffer solution containing 010 mol dm-3 of ethanoic acid and 020 mol dm-3 of sodium ethanoate. Calculate the pH of a solution that has a pOH of 870. 2- The chemical shift value of o-nitro toluene is higher than that of m-nitro toluene.
K a for ethanoic acid is 174 x 10-5 mol dm-3. Vinegar is at least 4 acetic acid by volume making acetic acid the main component of vinegar apart from water and other trace elements. Thus near the end point there is a rapid increase of pH from about 4 to 9.
Exothermic reactions release energy into the surroundings so they usually feel hot. Dissociation constant of weak acid acetic acid can be determined by finding the value of degree of. Learn more in this KS3 Chemistry guide from Bitesize.
Further of about 001 mL of 01 M NaOH will amount to adding hydrogen ions and the pH value will jump to about 9. Acetic acid glacial 100 CAS 64-19-7 anhydrous for analysis EMSURE ACSISOReag. Basicity increases with increasing of value on pH scale from 7 seven to 14 fourteen Thus by knowing the value of on pH scale strength of an acid or a.
The pH is then equal to minus the logarithm of the concentration value. What is the pKa value for natural indicators obtained from peonies poppies and red cabbage determined using. Now the acid is completely neutralized.
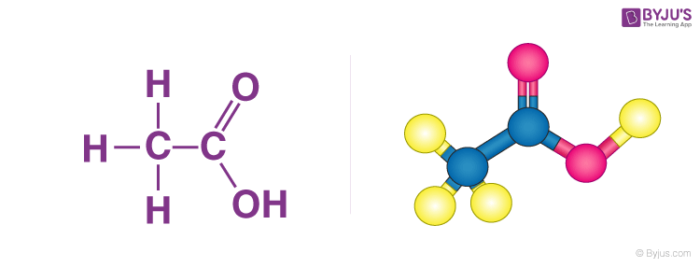
Acetic acid ə ˈ s iː t ɪ k systematically named ethanoic acid ˌ ɛ θ ə ˈ n oʊ ɪ k is an acidic colourless liquid and organic compound with the chemical formula CH 3 COOH also written as CH 3 CO 2 H C 2 H 4 O 2 or HC 2 H 3 O 2. What factors affect acid strength. Weak acids such as ethanoic acid CH 3 COOH do not fully dissociate.
Red pH 1-3 strong acid solution Orange pH 4-5 weak acid Pink pH 4 Yellow pH 6 weaker acid Green or pale green pH 7 neutral. Acidity increases with decreasing of value on pH scale from 7 seven to 0 zero. The strength of the H-A bond depends on that.
Transaconitic acid can be isolated during sugarcane processing by precipitation as the. Endothermic reactions are the opposite. PH log 10 H.
The mobile phase is generally an organic solvent such as hexaneheptane dichloromethane or ethyl acetate. Ethanoic acid citric acid present in citric fruits and acetic acid present in vinegar are a few examples of weak acids. In other cases the equivalence point.
Universal indicator test paper FLAM 1142 is mixture of acid-base indicators that causes a colour change for each change in pH value over a wide range. Under normal circumstances this means that the concentration of hydrogen ions in acidic solution can be taken to be equal to the concentration of the acid. Acetic acid concentration 000128 mol lit has an equivalent conductivity value of 004815 ohm-1 A.
Based on Polarity - n hexane cyclohexane trichloromethane. Strength of acid ie. The tighter the bond the more energy.
The lower values are obtained by adding sulfuric acid and higher values by adding solutions of ethanoic acid and ammonium sulfate or ammonium ethanoate. Aim To find the pH of the following samples by using pH paperuniversal indicator. And ethanoic acid.
These include acid and base models and definitions conjugate acid-base pairs and some basic acid-base reactions. Sometimes the pH value can be less than 0 for very strong acids or greater than 14 for very strong bases. A Aconitic acid 123-propenetricarboxylic acid C 6 H 6 O 6 CAS Reg.
In the formula above pH is the value we are looking for. Carborane comes with a pH value of -18. The concentration of the solution greatly affects the dissociation to form the hydrogen ion and the conjugate base acetate CH 3 COO At a concentration comparable to that in vinegar 10 M the pH is around 24 and only around 04 percent of the acetic acid molecules.
A Dilute hydrochloric acid b Dilute NaOH solution c Dilute ethanoic acid solution d Lemon juice e Water f Dilute sodium bicarbonate solution Theory pH is the measure of the hydrogen ion concentration H of a solution. Hydrochloric acid HCl is an example of a strong acid. PK is log value of the dissociation constantBecause it contains three acidic protons Phosphoric acid has three dissociation constants and each of the three can be used to create buffers for either of the three corresponding pH ranges.
Comparing the enthalpies of combustion of ethanol ethanal and ethanoic acid using calorimetry to quantify intermolecular force strength. As a rough rule of thumb the visible change takes place about 1 pH unit either side of the pK ind value. The exact values for the three indicators weve looked at are.
A mixture of hydrofluoric acid and pentafluoride antimony. Determining the pKa value for ibuprofen from pH curves using the half-equivalence method. Stationary phase is an alkylamine bonded to silica.
Their application to wool is similar to that for acid dyes but the pH value is restricted to. It is important that the learners have a good understanding of the work covered in Chapter 8 specifically equilibrium constants before studying this chapter. Normal Phase Liqiuid Chromatography The stationary phase is polar and the mobile phase relatively non-polar.
This makes acetic acid a monoprotic acid with a pKa value of 476 in aqueous solution. In fact only about 1 ethanoic acid. The pH chosen for the solution in the dyebath depends on the individual properties of the dyes.
Strength of base ie. By Public Water Supply System. 000499-12-7 occurs in the leaves and tubers of Aconitum napellus L.
Then all you have to do is to find the pH using. Ph Value Experiment Class 10 CBSE. Indicator pK ind pH range.
Ph Eur - Find MSDS or SDS a COA data sheets and more information. The three pKa values for phosphoric acid are 215 686 and.

To Find The Ph Of The Samples By Using Ph Paper Universal Indicator Lab Work

Ethanoic Acid Properties Structure Uses Reactions And Faqs Of Ethanoic Acid
What Is The H Of A Solution Of Ethanoic Acid With A Ph Of 3 9 Quora

No comments for "Ph Value of Ethanoic Acid"
Post a Comment